TamaBio aims to provide optimal
solutions for
patients through
product development using
cutting-edge technology.
With Aichi Children’s Health and Medical Center, TamaBio launches Clinical Observational Study Using “PeriBeam®” Pericardial Membrane.
PeriBeam®, the FDA listed Pericardial Membrane, manufactured by TamaBio, a FDA registered establishment, was exported to the United States for the first time this week.
At TERMIS-AP 2025, the possibility of our technology being used to treat myocardial infarction was mentioned.
The artificial dura mater “DuraBeam®” will be exhibited at the 84th Annual Meeting of the Japan Neurosurgical Society (Wednesday, October 29th, 2025 – Saturday, November 1st, 2025).
We will be exhibiting the artificial pericardium prosthesis “PeriBeam®” at The 78th Annual Scientific Meeting of the Japanese Association for Thoracic Surgery (2025) (Thursday, October 23rd to Saturday, October 25th, 2025).
TamaBio will be sponsoring the 36th Japanese Society of Diencephalo-Pituitary Tumors (Thursday, March 5th and Friday, March 6th, 2026, Fukuoka City, Japan).
TamaBio has received a notice of grant decision for subsidy to support the international patent application for our domestic joint patent inventions with Tohoku University [Regenerative Medical Devices – Brain Tissue etc. Damage Repair Sheet].
TamaBio will be sponsoring the 57th Spine Surgery Club Meeting to be held on Aug.23rd at Nagasaki.
Nippon Medical School and TamaBio Sign New Joint Research Agreement : Regenerative Therapy for Infarcted Myocardium Using Epicardial Approach
Tamabio Co., Ltd. opened an account on “X”.
Biocompatibility and Tissue Regeneration Ability of the artificial dura mater DuraBeam was presented at CNTT2025/JSAN2025, Osaka International Convention Center
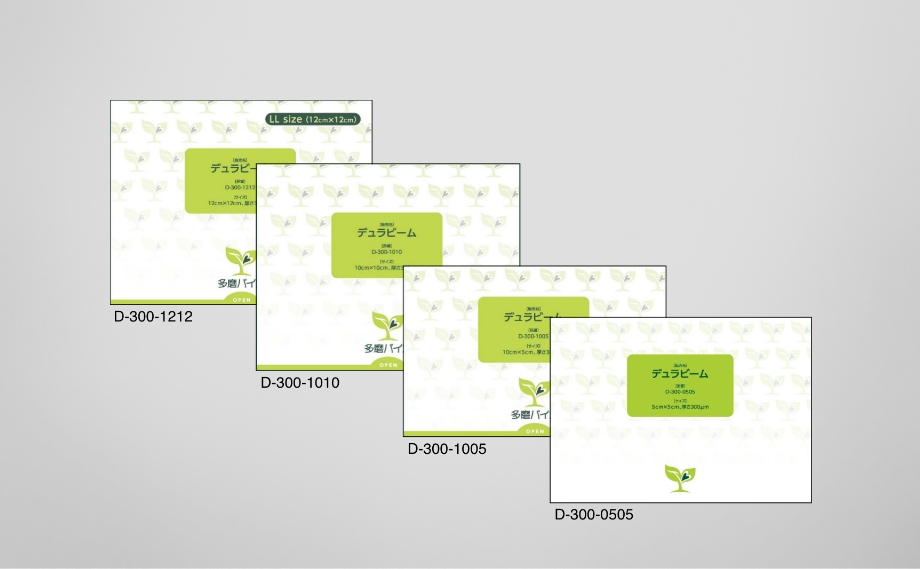
DuraBeam®︎
- Classification
Highly-regulated medical device (Class IV)
- Purpose of use/effect
Brain dura mater replacement and substitution
- Sizes
120mm×120mm,100mm×100mm,
100mm×50mm,50mm×50mm- Thickness
0.30mm(300μm)
Medical device approval number: 22900BZX00291000

PeriBeam®︎
- Classification
Highly-regulated medical device (Class IV)
- Purpose of use/effect
Pericardial replacement and substitution
- Sizes
120mm×120mm,100mm×100mm
- Thickness
0.09mm(90μm)
Medical device approval number: 23000BZX00360000

TamaBio : Contributing to medical care through innovative technology
TamaBio was established in April 2016 using RIKEN, the National Research and Development Agency’s special processing technology for polymer resin as a practical basic technology. TamaBio has so far obtained manufacturing and sales approval (Class IV : Highly Controlled Medical Device) from the Ministry of Health, Labor and Welfare for the Dura Mater Substitute “DuraBeam®” and the Pericardial Membrane “PeriBeam®”, and has obtained core patents in Japan, The United States, The People’s Republic of China, and Taiwan. The products are distributed by major specialized medical device companies to medical institutions and hospitals. Tamabio's synthetic artificial biomembrane has been used clinically in thousands of surgeries to date and is expanding.
The essence of TamaBio's artificial biotechnology is to realize the development of medical devices that promote the regeneration of human organ tissues and cells. TamaBio's products have been shown to be highly biocompatible and to bring about biological tissue repair through thousands of clinical use results to date. TamaBio has obtained patents for nerve damage repair sheets, brain damage repair sheets, and spinal cord injury repair sheets in collaboration with Tohoku University (the patent was registered in 2024). TamaBio will continue to forge ahead with the development of medical devices that can repair and regenerate patients' lost tissue.
- Company name
- TamaBio Company Limited
- Established
- 2016/4/21
- Head Office
- 402 Gran Creste 2-2-18 Sakai Musashino-shi Tokyo 180-0022 Japan
- Business
- Medical device manufacturing / Class 1 manufacturing and sales Development, manufacturing, and sales of Class IV highly controlled medical devices (dura mater and pericardial prosthesis material) Development, manufacturing, and sales of regenerative medical devices and other synthetic artificial biomembranes.
- Managing
Director / CEO - Tetsuya Nagao
- TEL / FAX
- TEL : +81-422-53-5051
/ FAX : +81-422-38-5091
TamaBio is promoting the development of Dura Mater Substitute “DuraBeam®︎”, Pericardial Membrane “PeriBeam®︎”, and artificial organs and regenerative medicine technologies in more critical and promising fields. Medical devices such as implants are being developed around the world as functional substitutes for patients’ lost organs, but in clinical settings, various adverse events such as postoperative infections, adhesion with self-tissue, calcification due to long-term use and leakage / blockage of bodily fluids are issues. TamaBio products are highly biocompatible, and overcome these issues through its processing technology, and in recent years have been used as permanent implantation devices or short-term use devices in many surgeries.
The important thing about TamaBio's technology is that it regenerates tissues in the human body, that is, it regenerates important cells and tissues as a whole to maintain their health and restore organ function. It is a technology to produce highly biocompatible polymer compounds, and to process biomembranes to give them the ability to regenerate damaged tissue after implantation.
In addition to the latest developments in nerve injury repair sheets, brain injury repair sheets, and spinal cord injury repair sheets, important research results are being produced daily in the field of cardiovascular surgery and other fields. We would like doctors, researchers, investors, distributors, experts, and managers to participate in TamaBio and deliver cutting-edge technology to the world and to patients.
Tetsuya Nagao

General Manager under the Pharmaceutical and Medical Devices Act:
Manufacturing & Distribution, Quality Control
Ex McKinsey & Company
MSc, Aerospace Engineering, The University of Tokyo,
(Osuga/Hori Laboratory, AI-assisted Aircraft Design System Development)
BS from the Department of Aeronautics, The University of Tokyo
(Sato Laboratory, Research on “Riblets”, Relaminarizing Turbulent Flows)