
PeriBeam®︎
This product was developed by TamaBio Co., Ltd. based on medical sheet technology patented in Japan, the United States, the People’s Republic of China, and Taiwan, and has received regulatory approval.
- Product Name
-
PeriBeam®︎
- Approval number
-
23000BZX00360000
- Device Common Name
-
Prosthetic material for artificial pericardium
- JMDN code
-
36182000
- Sterilized
-
single use
- Product number
Product Sizes
Product Sizes - P-090-1212
LL size
12cm×12cm thickness 90μmJAN Code4589795950234
P-090-1010L size
10cm×10cm thickness 90μmJAN Code4589795950227
| Product number | P-090-1212 | P-090-1010 |
|---|---|---|
| Product Sizes | LL size | L size |
| JAN Code | 4589795950234 | 4589795950227 |
*This product uses a patent from RIKEN a National Research and Development Institute.
Manufacturer: TamaBio Company Limited
402 Gran Creste 2-2-18 Sakai Musashino-shi Tokyo 180-0022 Japan
TEL : +81-422-53-5051
FAX : +81-422-38-5091
1.Product overview
[Product Name] PeriBeam®︎
[Category] Medical supplies 04 Orthopedic supplies
[General name] Prosthetic material for artificial pericardium
[Class classification] IV
[Date of approval] November 26, 2018
[Approval number] 23000BZX00360000
[Purpose of use or effect] Pericardium replacement and substitution
[Structure/Principle]
This product is a sheet made of expanded polytetrafluoroethylene (ePTFE), and one side is irradiated with an ion beam. The ion beam irradiated surface has a slightly brown color, and the non-irradiated surface is white and smooth, so it is possible to distinguish between the irradiated and non-irradiated surfaces.
For differentiation, “Ion”mark is stamped on the ion beam irradiated surface. The thickness is 90μ (0.09mm).
Development history/development concept
・As a prosthetic material for artificial pericardium, reducing the risk of adhesions and infection
As a bioprosthetic material, ePTFE is a material that is extremely stable and does not deteriorate in the body, but the ePTFE sheets that have been used for pericardial defects have low tissue affinity and are at risk of infection. It is said that many cases require reoperation. On the other hand, biologically derived pericardial sheets have been widely used, but they are said to be dangerous as they have been observed to become brittle due to the influence of preservation solutions as well as calcification.
In this product, the ion beam irradiated surface of the ePTFE sheet is processed to be porous, allowing tissue cells to enter and form a membrane, which is thought to reduce the risk of infection. Furthermore, it is thought that the risk of adhesion will decrease if a membrane is formed. This product can be attached to living tissue via fibrin glue.
・Easy to remove by reoperation
Although this product adheres to living tissue as described above, no bad adhesion to the tissue was observed, suggesting that it is easy to remove during reoperation.
・Soft fit to the body
Manufactured using a unique molding method of multi-axial stretching, it has an outstanding feel and softness.
In addition, it has been confirmed that it has sufficient strength through accelerated fatigue tests, strength tests, suture tensile strength tests, burst tests, etc. assuming heartbeat.
・Development history
RIKEN a National Research and Development Institute, Tokyo Women’s Medical University, and the Institute of Chemistry and Serum Therapy conducted research into preventing postoperative cerebrospinal fluid leakage by surface modification through ion beam irradiation (roughening the ePTFE surface to improve cell adhesion).
Four types of monovalent positive ions (4He+, 20Ne+, 40Ar+, 84Kr+) were irradiated onto ePTFE, and a comparative study of biocompatibility was performed on the dura mater. In order to analyze the adhesion and sealing effect between the ion beam irradiated ePTFE and the dura mater, a test of embedding samples into rabbit dural defects was performed. As a result ion irradiated samples were firmly attached to the dura mater and skull of the living body immediately after fibrin glue was applied. Furthermore, in a 1-month indwelling test of a sample in a rabbit dural defect, no cerebrospinal fluid leakage was observed with ion beam irradiated ePTFE compared to non-irradiated ePTFE.
(Cited document: Journal of the Japanese Endocrine Society Vol.80 Sep. 2004)
Cross-sectional electron micrograph (×1000)
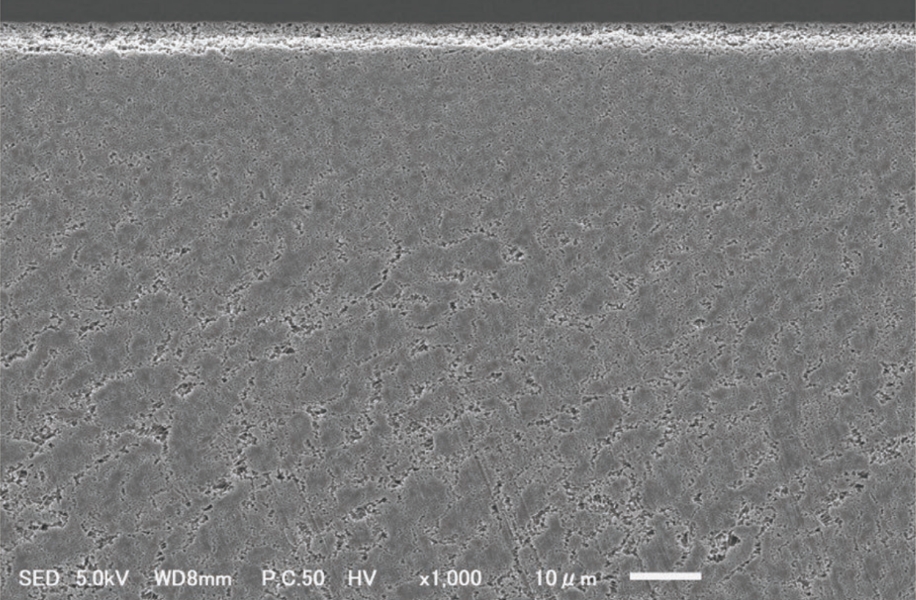
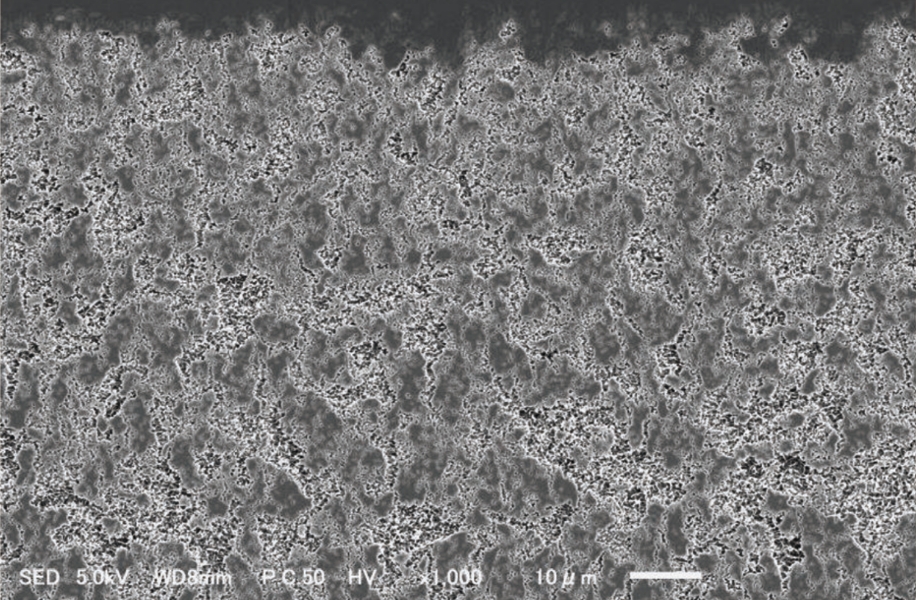
- Ion beam irradiation causes unevenness on the irradiated surface.
- Etching processing by sputtering
Porcine pericardial implantation test of PeriBeam®︎
[Implantation method] Using 10 pigs, this product was transplanted into the pericardial membrane and autopsied to confirm its efficacy and safety.
[Group 1 (PeriBeam®︎/external facing)] Implanted with the ion beam irradiated surface facing outward (pericardial membrane side) and the smooth surface facing inward (heart side).
[Group 2 (PeriBeam®︎/inward facing)] Implanted with the ion beam irradiated surface facing inward (heart side) and the smooth surface facing outward.
[Group 3 (control/similar medical device)] Both the inner and outer surfaces are smooth.
[Observation period] 8 weeks after implantation.
[Test results] No defects or adverse events were observed in all cases.
Porcine pericardial implantation test: 56 days later
When a 90 μm thick ePTFE sheet irradiated with an Ar+ ion beam was implanted into the pericardial membrane of a pig, two months later a new membrane was formed on the ion beam irradiated surface, as shown in the white frame in the photo below.
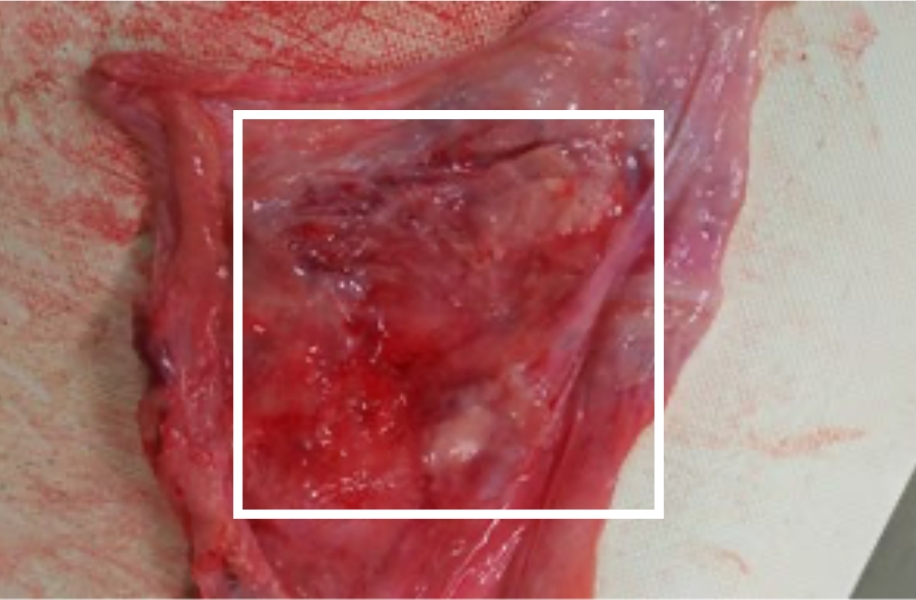
As seen in this pathological specimen, the surface of the ePTFE sheet irradiated with the ion beam was infiltrated with lymphocytes and macrophages, and further proliferation of fibroblasts was
observed.

4.Photo of this product
Ion beam irradiated surface
It is stamped with “ION” or “Ion”. It has a matte white color with a slightly brownish color.

Ion beam non-irradiated surface
White, smooth, and slightly glossy.
